Which of the Following Ground-state Ions Has Unpaired Electrons
N i 3 has A r 3 d 7 electronic configuration. If none enter a zero in the answer box.
7 How many valence electrons does magnesium have.

. The elements with one unpaired electron in their ground state electron configuration from the atomic number 1 to the atomic number 36 are. 11 What is meant by. Z n 2 has A r 3 d 1 0 electronic configuration.
23 which of the following ground state ions has the. So you have um the nitrogen oxygen um phosphorous ion and the night the sodium ion. I can see that Ca has an unpaired electron but it doesnt in the ground state right.
A First write down the ground state configuration of Sb. Al O Ti2 N s2- N. Hydrogen H lithium Li Boron B Fluorine F sodium Na aluminum Al chlorine Cl potassium K silicon Sc copper Cu gallium Ga and bromine Br.
Ti 31s 22s 22p 63s 23p 63d 1. Thus we see that Fe 2 has the most unpaired electrons. Valence electrons are the electrons utilised for bonding.
Which element has the following ground-state electron configuration. C u has A r 3 d 1 0 electronic configuration. Note d has 5 orbitals and each orbital can have up to 2 paired opposite spin electrons all are paired at d10 and s also has 2 paired opposite spin electrons.
A Cr2 B Mn2 C Ni2 DCu E CO2. Atomic size generally follows certain periodic trends and these trends can be used to predict relative size. 10 What is the electron configuration of magnesium.
Pages 7 This preview shows page 6 - 7 out of 7 pages. This problem has been solved. Course Title ENGLISH MISC.
The question here asked How many impaired electrons are there in the following ground state atoms or ions. Iv ceB2 has 10 electrons in its structure and its electronic configuration is. So in order to determine this you need to first of all drought this Elektronik configuration in the off ball principle.
6 How many unpaired electrons does s have. The electrons are first removed from valence shell and then inner shells. Show transcribed image text.
What is the expected number of unpaired electrons in the ground state electron configuration of iodine. Ceσ1s2 σ 1s2 σ 2s2σ 2s2 π 2p_y1 π 2p_z1. V 31s 22s 22p 63s 23p 63d 2.
B How many unpaired electrons does iodine have. Which of the following ground-state ions has unpaired electrons. 8 How many energy levels does Mg have.
A ground-state atom of manganese has ___ unpaired electrons and is ___. Mg 21s 22s 22p 6. Sc2 Which of these has the smallest radius.
Which of these ground-state ions has unpaired electrons. _____ arises when the unpaired electrons of the atoms or ions in a solid are influenced by the orientations of the electrons of their neighbors asked Jul 29 2018 in Chemistry by Hogwarts general-chemistry. Which of these ground-state ions has unpaired electrons.
Hence the structure of boron has two unpaired electrons. Kr4d10 5s2 5p3 then your removing 3 3 electrons from the ground state configuration so you will have Kr4d10 5s2 so there is 0 unpaired electrons. Which of the following atoms or ions has three unpaired electrons.
An excited state configuration is a higher energy arrangement it requires energy input to create an excited state. 4 How do you know how many unpaired electrons. F e 2 ion Z26 has electronic configuration A r 3 d 6 4 s 0.
The answer is one. It contains 3 unpaired electrons. N has 3 unpaired electrons.
Select True or False Sodium ions are more reactive than sodium atoms The or False True False An element with the general electron configuration for its outermost electrons of ns2pl would Multiple Choice ЗА 4А 8А whey 5A 2A. Given the following electronic configuration of neutral atoms identify the element and state the number of unpaired electrons in its ground state. F N S 2 Mg 2 Sc 3 Ti 3.
Which of these ground-state ions has the largest number of unpaired electrons. So we know that um nitrogen here is going toe have Its. Multiple Choice 2- Sc2- Mg24 p3 15.
Which of the following ground-state ions has the largest numberof unpaired electrons. See the answer See the answer See the answer done loading. 23 Which of the following ground state ions has the largest number of unpaired.
ANe BCa CNa DO2-The answer is B but I thought it was Na as Na ground state has an unpaired e-. Na has 0 unpaired electrons. Which of the following ground-state ions has unpaired electrons.
5 Does MN 3 have unpaired electrons. Which one of these ions does not have Kr as its electronic configuration. The sulfide ion 2- is isoelectronic with which one of the following.
9 Which of the following has two unpaired electron. It contains 0 unpaired electrons. Up to 256 cash back Get the detailed answer.
Thus F e 3 have highest number of unpaired electrons. The largest number of unpaired electrons. If we consider this then M n 3 F e 3 C o 3 and N i 3 have 4 5 4 3 unpaired electrons respectively.
A B P3- 75 2 C Mg2 D ScZ 52. It contains 4 unpaired electrons. It contains zero unpaired.
Hence the peroxide ion ceO2-2 does not contain any unpaired electrons. A B P3- 75 2 C Mg2 D ScZ 52. 17 Which of the following species has an unpaired electron in its ground-state electronic configuration.
Which of the following ground-state ions has unpaired electrons.

Solved 25 What 2 Ion Has The Following Ground State Chegg Com
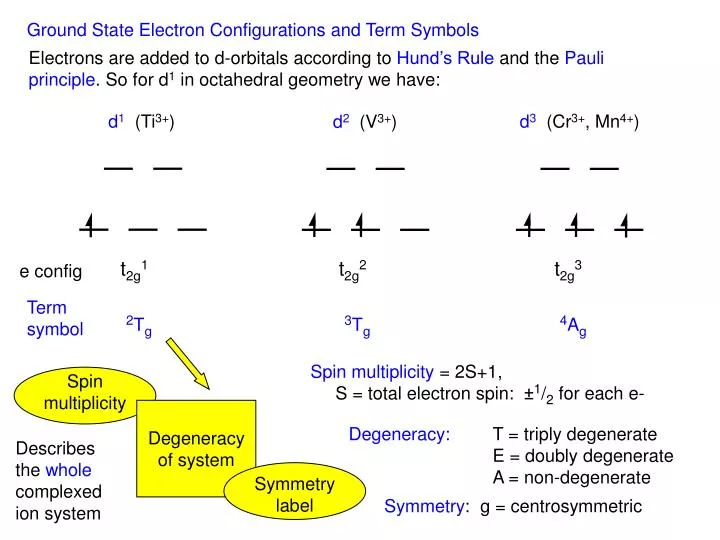
Ppt Ground State Electron Configurations And Term Symbols Powerpoint Presentation Id 2043186
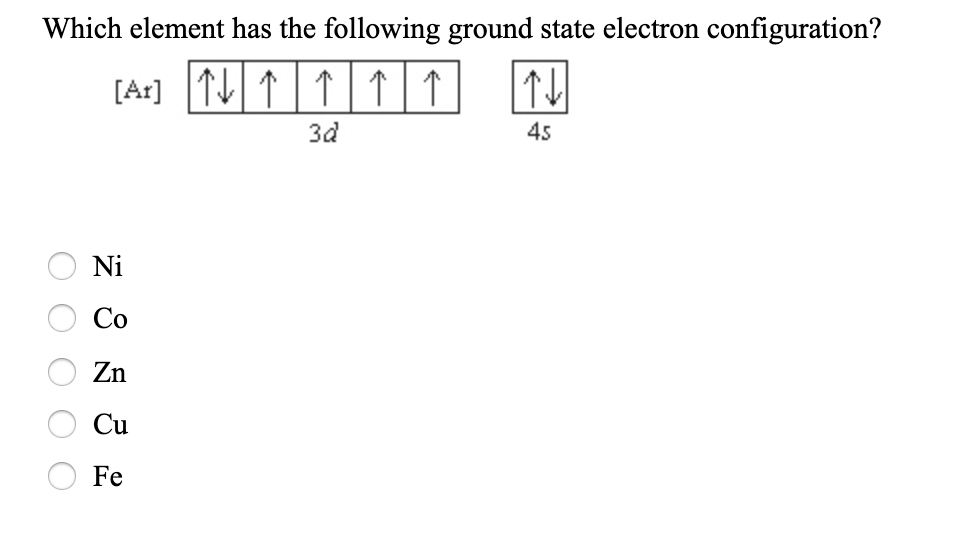
Answered Which Element Has The Following Ground Bartleby
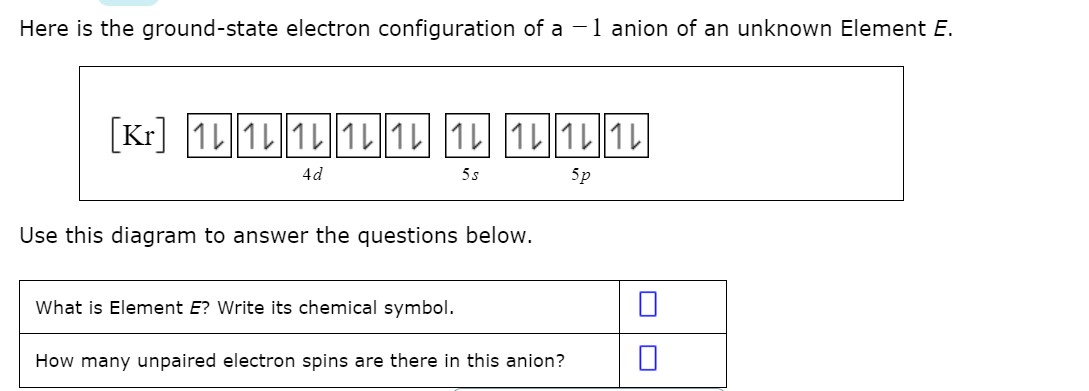
Answered Here Is The Ground State Electron Bartleby

Solved Write Ground State Electron Configurations For The Chegg Com
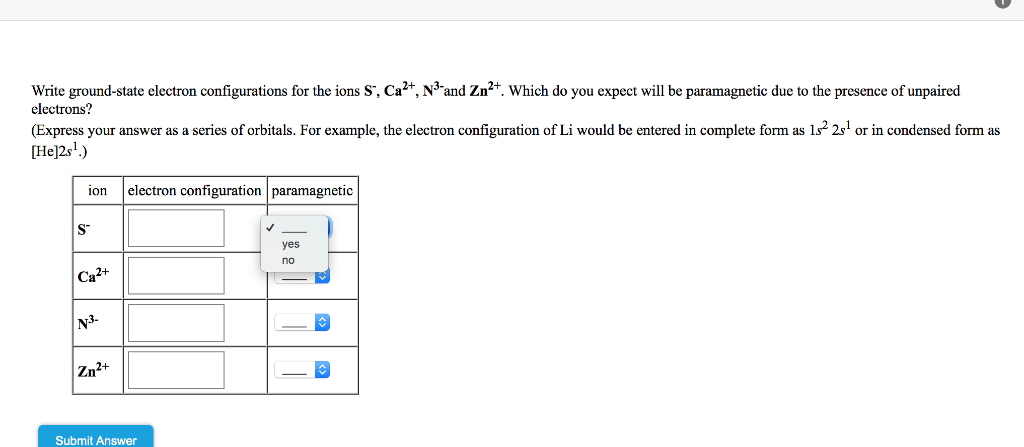
Solved Write Ground State Electron Configurations For The Chegg Com
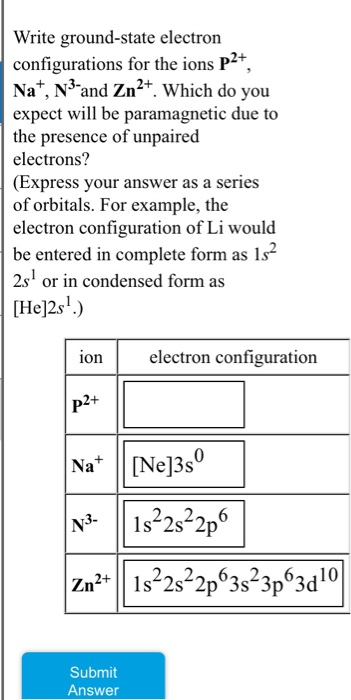
Solved Write Ground State Electron Configurations For The Chegg Com

Solved Which Of The Following Ions Have The Same Ground Chegg Com

Solved Give The Ground State Electron Configuration And Chegg Com
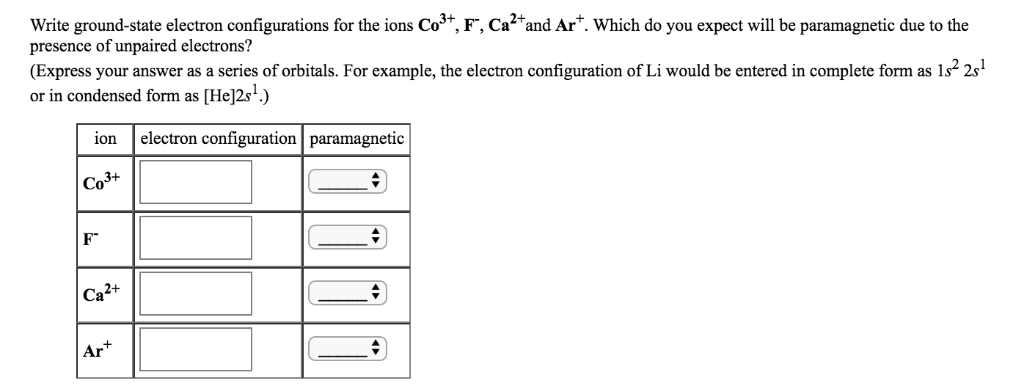
Solved Write Ground State Electron Configurations For The Chegg Com

Solved 24 Which Of The Following Ions Have The Same Ground Chegg Com

For Each Atom Ion Provide The Condensed Ground State Electron Configurations The Box Diagram Of The Valence Electrons And The Number Of Unpaired Electrons S N Cr Cr3 Fe2 N3 As Study Com

Solved Write Ground State Electron Configurations For The Chegg Com
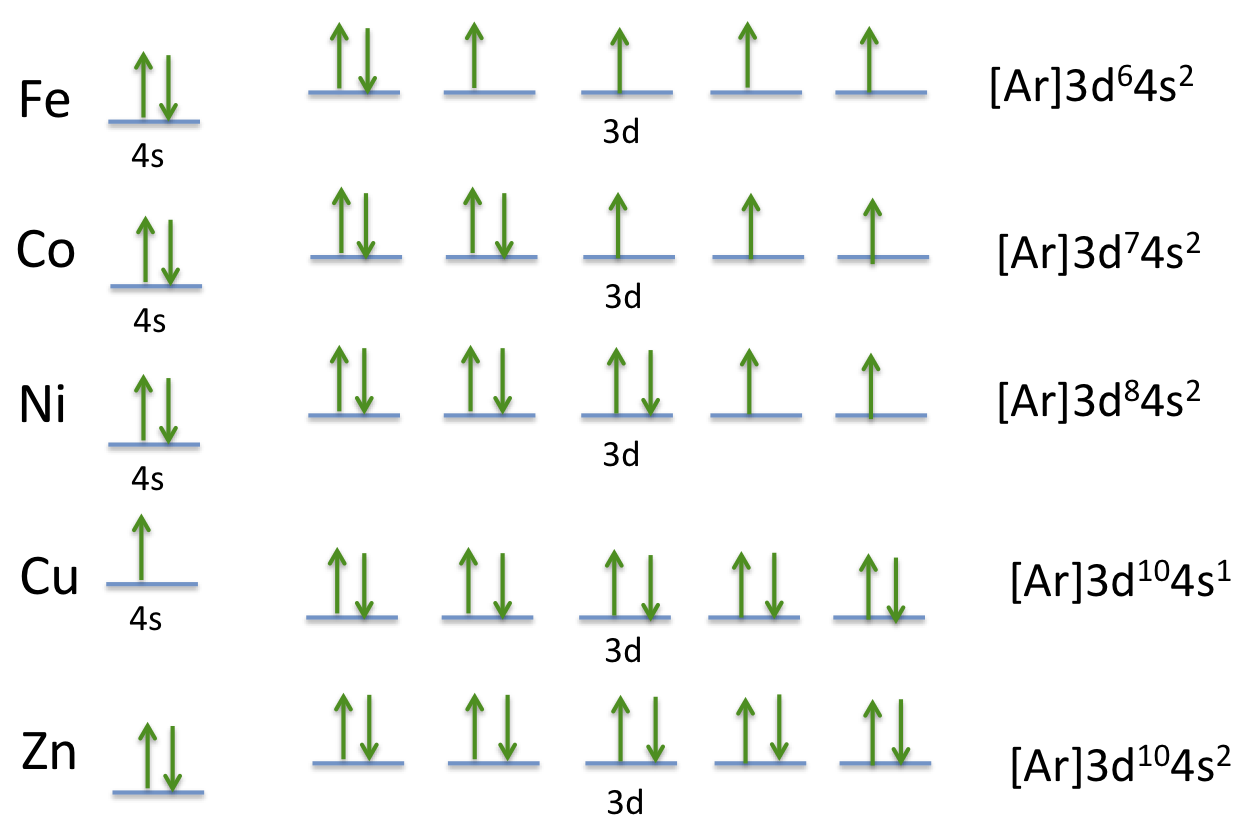
How Many Unpaired Electrons Are In An Atom Of Co In Its Ground State A 7 B 1 C 3 D 2 Socratic
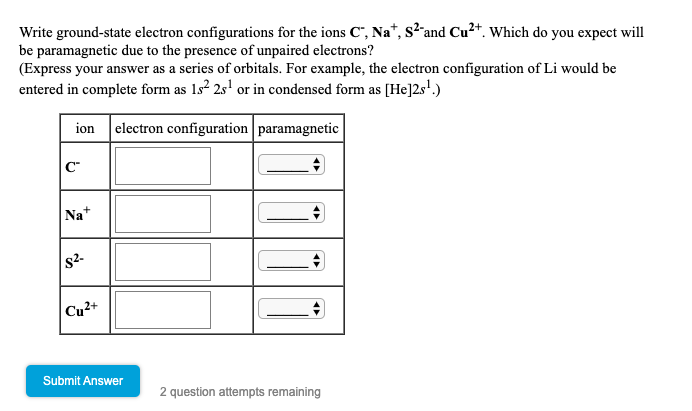
Solved Write Ground State Electron Configurations For The Chegg Com
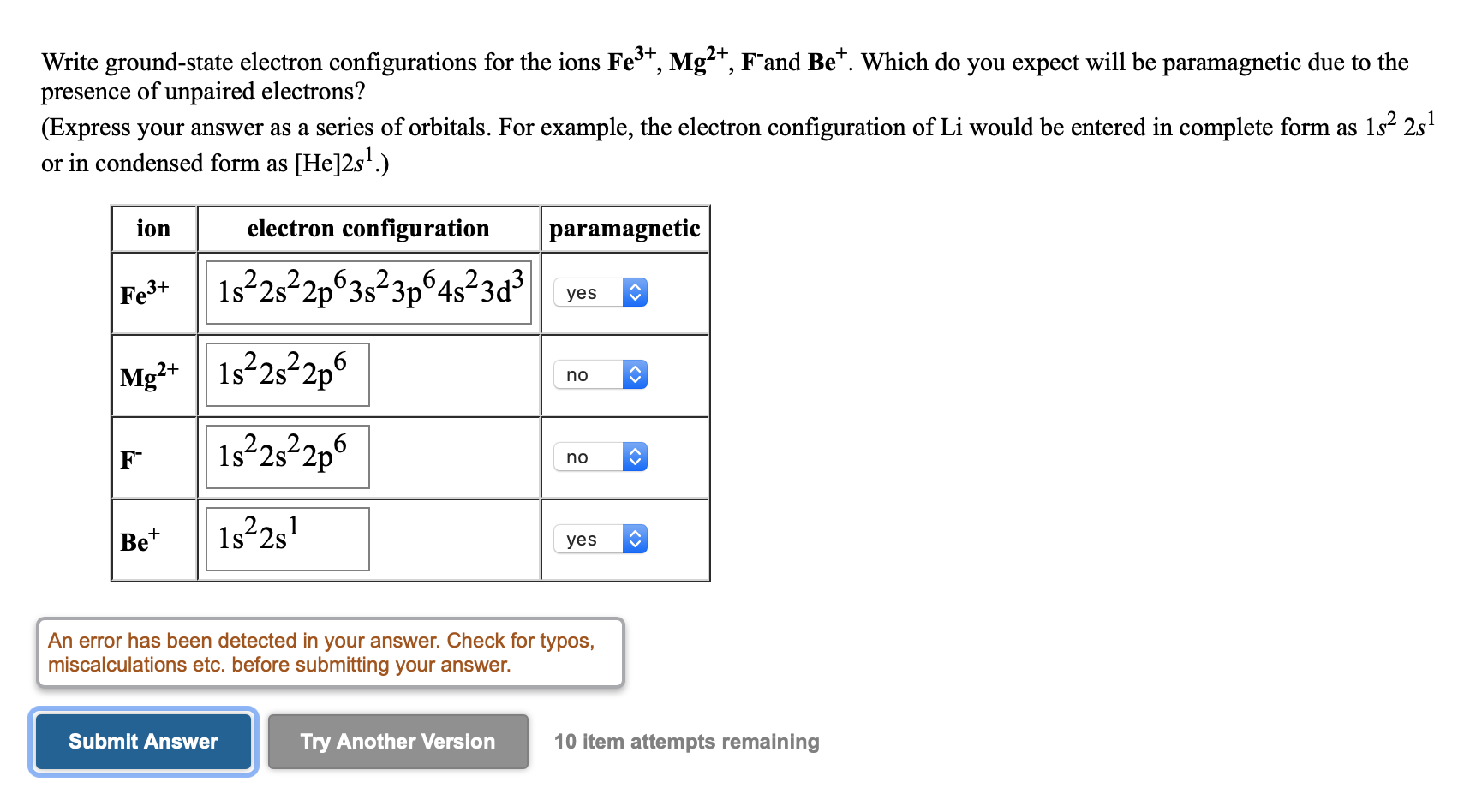
Solved Write Ground State Electron Configurations For The Chegg Com

In The Ground State Of Cobalt Atom Z 27 There Are Unpaired Electrons Youtube

Write Ground State Electron Configurations For The Ions Bek O And Fe2 Which Do You Expect Will Homeworklib
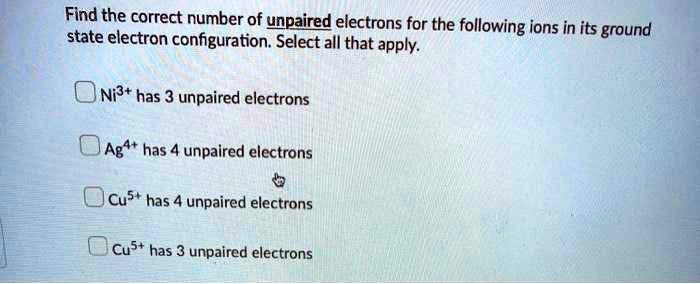
Solved Find The Correct Number Of Upaired Electrons For The Following Ions In Its Ground State Electron Configuration Select All That Apply Ni3 Has 3 Unpaired Electrons Ag Has 4 Unpaired Electrons
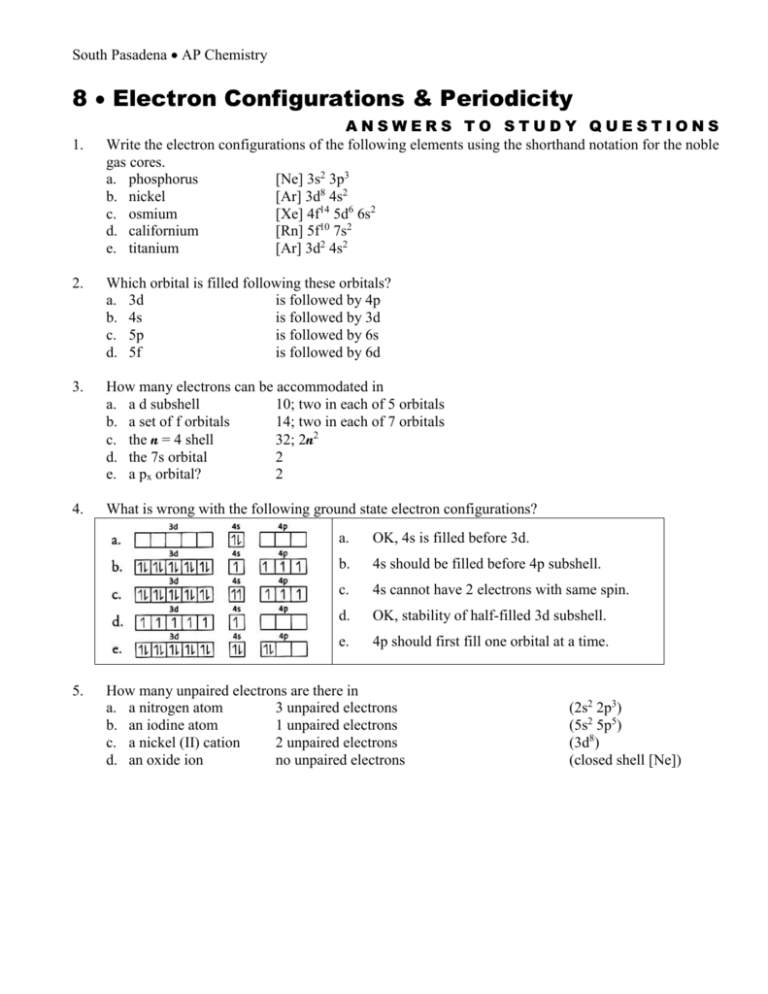
Comments
Post a Comment